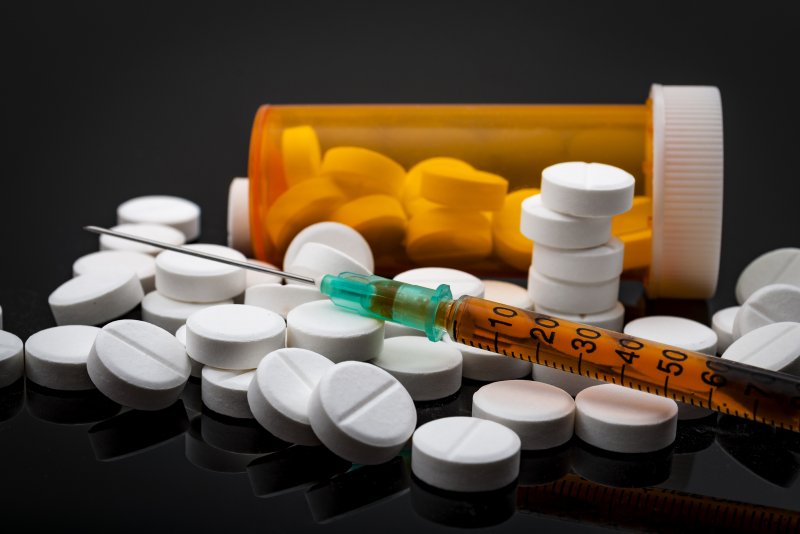
The first generic naloxone nasal spray to treat opioid overdose has received approval from the U.S. Food and Drug Administration.
Teva Pharmaceuticals' lifesaving product is also the first generic naloxone nasal spray approved for use by people without medical training. There was already a brand-name spray, Narcan, for emergency use by untrained people, such as family members and bystanders.
The need is urgent. On average, more than 130 Americans die every day from overdoses of opioids -- including prescription painkillers such as fentanyl, oxycodone [OxyContin], hydrocodone (Vicodin) and morphine, as well as illegal drugs such as heroin or drugs sold as heroin, the FDA said.



 More than 100 substances widely used in common US foods, supplements and beverages underwent no health...
More than 100 substances widely used in common US foods, supplements and beverages underwent no health... Last month, Justin and Amy Miller packed their vehicles with three kids, two dogs, a pet...
Last month, Justin and Amy Miller packed their vehicles with three kids, two dogs, a pet... A respiratory virus that doesn’t have a vaccine or a specific treatment regimen is spreading...
A respiratory virus that doesn’t have a vaccine or a specific treatment regimen is spreading...






























