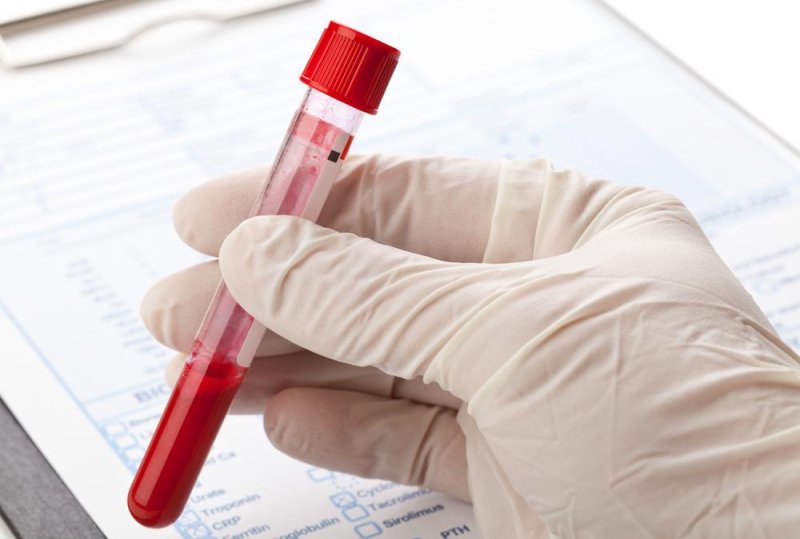
 A San Diego company selling an early cancer detection test was notified by the U.S. Food and Drug Administration it can find no evidence the test actually works, and is concerned it could prove to be harmful for some people.
A San Diego company selling an early cancer detection test was notified by the U.S. Food and Drug Administration it can find no evidence the test actually works, and is concerned it could prove to be harmful for some people.
Pathway Genomics debuted its CancerIntercept test in early September with claims it can detect cancer cell DNA in the blood, picking up mutations linked to as many as 10 different cancers. The goal is to catch cancer early in people who are "otherwise healthy" and not showing symptoms of the disease.
"Based on our review of your promotional materials and the research publication cited above, we believe you are offering a high risk test that has not received adequate clinical validation and may harm the public health," said FDA Deputy Director James L. Woods in a letter to the company.
CancerIntercept is billed by the company as a blood test looking for DNA fragments in the bloodstream and testing them for 96 genomic markers it says are found in several specific tumor types.



 The Food and Drug Administration Monday unveiled the details of a new policy designed to make...
The Food and Drug Administration Monday unveiled the details of a new policy designed to make... A group of 14 law firms representing nearly 20,000 plaintiffs is seeking to intervene in Bayer’s...
A group of 14 law firms representing nearly 20,000 plaintiffs is seeking to intervene in Bayer’s... As marijuana use among teens has grown in the past decade, researchers have been trying to...
As marijuana use among teens has grown in the past decade, researchers have been trying to...






























