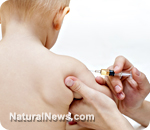
 An unlicensed vaccine being quietly shipped into the U.K. from the Czech Republic is sparking controversy as it may be linked to causing severe allergic reactions. Medi-Mumps, a single mumps vaccine cultured from dog kidney cells, is being touted by some as an alternative to the controversial combination measles, mumps and rubella (MMR) vaccine, but others have major concerns about both its source and its potential for triggering severe canine allergies.
An unlicensed vaccine being quietly shipped into the U.K. from the Czech Republic is sparking controversy as it may be linked to causing severe allergic reactions. Medi-Mumps, a single mumps vaccine cultured from dog kidney cells, is being touted by some as an alternative to the controversial combination measles, mumps and rubella (MMR) vaccine, but others have major concerns about both its source and its potential for triggering severe canine allergies.
Back in 2002, the Committee on the Safety of Medicines, an independent U.K. advisory committee that evaluates the quality, efficacy and safety of medicines, rejected a single mumps vaccine known as Pavivac because of serious safety concerns. Just like Medi-Mumps, Pavivac was made from dog kidney cells in the Czech Republic, but was considered potentially hazardous with not enough evidence to back up claims that it was safe and effective.
Now it appears as though Pavivac has simply been repackaged and rebranded as Medi-Mumps, which similarly lacks proper safety data showing that it is effective with minimal risk. And yet parents opting for single vaccines for their children will likely be presented with Medi-Mumps as a viable alternative to MMR, even though it could cause the very same harm as MMR.



 The U.S. government has given an ultimatum to the international group that helps provide vaccines to...
The U.S. government has given an ultimatum to the international group that helps provide vaccines to...






























